What We Do
We are dedicated to understanding how cellular signaling is organized and executed on membrane surfaces. By employing in-vitro/in-situ cryo-electron microscopy (cryo-EM), biochemistry, and cell biology, we aim to unravel how membrane surfaces govern protein function in physiologically relevant conditions.
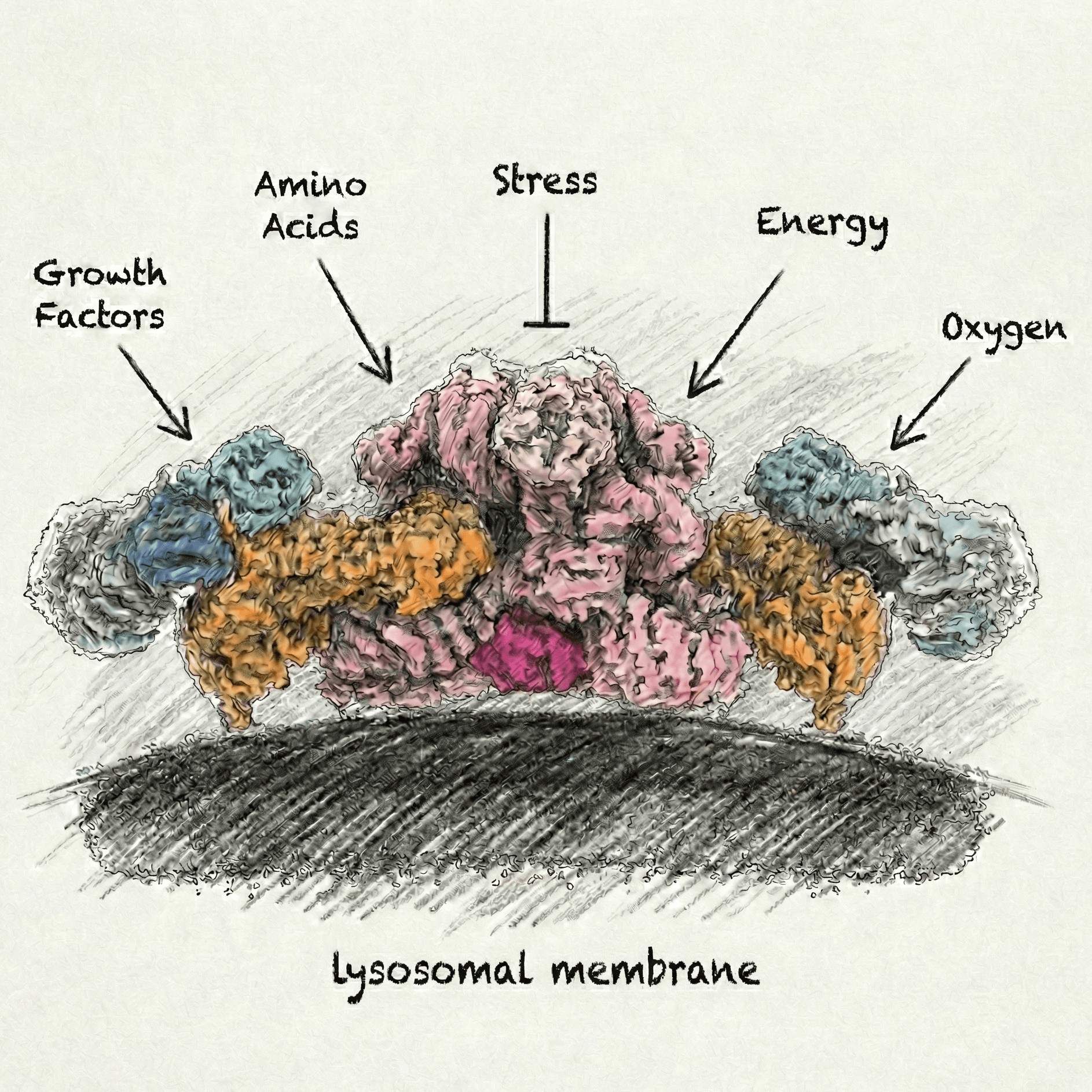
The Lysosome: A Signaling Hub
Far beyond its role in degradation, the lysosome functions as a critical sensing platform. Its membrane is the physical interface where multiple signaling inputs converge. At the heart of lysosomal signaling pathways is the mTORC1 (mechanistic target of rapamycin complex 1), which serves as the master regulator of cellular growth and metabolism. Our research explores how the lysosomal membrane acts as a sophisticated command center where nutrient availability and growth factor signals are integrated to control mTORC1 activity. We are specifically focused on the upstream "logic" that governs mTORC1 activation. A primary interest is understanding how growth factor signals ultimately converge with nutrient signals at the lysosomal membrane. To decode these processes, we utilize in vitro reconstitution, biochemical and biophysical characterization, and high-resolution cryo-electron microscopy (cryo-EM) to delineate these complexes in states that mimic their native membrane environment.
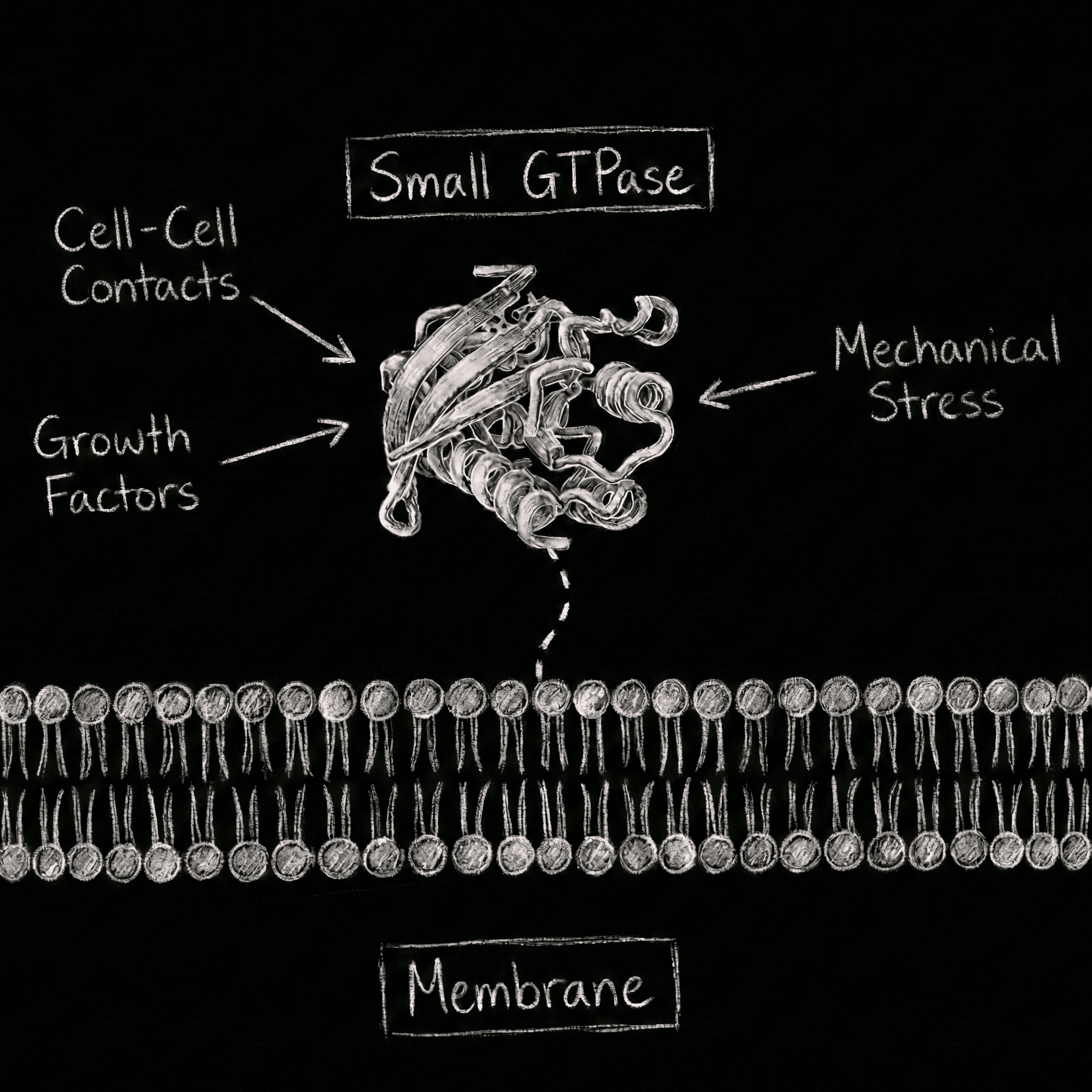
Small GTPases on Membranes
Small GTPases function as fundamental molecular switches that integrate environmental cues to coordinate cellular growth, metabolism, and adaptation. Our lab investigates the spatiotemporal regulation of these switches, focusing on how the membrane surface directly influences the interaction between signaling molecules. Our initial focus will be on the RAS GTPases, which are central to human health, with mutations implicated in nearly 20% of all human cancers. We aim to unravel the molecular and structural basis of RAS signaling on the plasma membrane. In addition, we combine AI and structural biology to guide drug discovery toward membrane-specific druggability. While RAS is a primary focus, our lab also explores the broader field of small GTPases to identify universal principles of membrane-directed signaling.

Membrane Remodeling
Biological membranes are far more than static barriers; they are highly dynamic, living interfaces that coordinate the intricate signaling networks of the cell. Membrane remodeling is a continuous and ubiquitous process essential for life. In a healthy cell, membranes are under constant physical and chemical transformation to facilitate essential processes, including vesicular traffic, organelle maintenance, and stress response. The "logic" of these remodeling events is often driven by specialized proteins that sense lipid identity and membrane curvature. Our lab seeks to bridge the gap between the basic mechanistic insights of membrane remodeling and their application in therapeutic intervention.